HETEROKARYON FORMATION
TAMOTSU OOTAKI
In Phycomyces. E. Cerdá-Olmedo and E. D. Lipson (eds.), 1987, p. 345-349. CSH Laboratory Press, Cold Spring Harbor, NY, USA.
Heterokaryosis is the existence of two or more genetically different types of nuclei in the same cell. Heterokaryon formation is essential for genetic complementation testing. The almost totally nonseptate and coenocytic nature of Phycomyces hyphae make them especially useful for complementation studies. Heterokaryons may originate by (1) somatic mutation of some of the nuclei present in the coenocyte, (2) fusion between genetically different cells, and (3) inclusion in a germspore of different meiotic products.
Heterokaryons formed naturally in the sexual cycle (Blakeslee 1906; Burgeff 1914, 1928; Orban 1919) or from spontaneous mutations in vegetative mycelia are not practical for routine genetic analyses, especially for complementation tests.
The anastomosis or fusion of vegetative hyphae is very rare in Phycomyces, although it is frequent in Ascomycetes and Basidiomycetes. Protoplast fusion has been demonstrated in Phycomyces by Binding and Weber (1974) and by Suarez et al. (1985); the currently recommended method is described in Appendix 4. Surgical "forced" techniques have been developed to make viable heterokaryons from normal cells. These techniques have several variants that are all based on exceptional characteristics of Phycomyces: the large size of its sporangiophores and its amazing capacity to regenerate sporangiophores, hyphae, and even protoplasm.
Protoplasm Mixing
Two stage I sporangiophores are plucked from mycelia of different genetic type, placed side by side in the bottom of a petri dish, and cut together in halves. The protoplasm is squeezed out from the upper halves of both sporangiophores and then mixed to form a single droplet. Pieces of wet paper are then immediately placed in a small circle around the mixture to retain high humidity, and the petri dish is covered and stored in the dark. Weide (1939) and Heisenberg and Cerdá-Olmedo (1968) have obtained heterokaryons using this method.
Recently, I have (unpubl.) improved this technique by using the hanging drop culture method (see Fig. 1).

Figure 1. Hanging-drop method for culturing the extruded protoplasm from two genetically different strains in Phycomyces. Heterokaryotic hyphae sometimes appear from the mixture of protoplasm.
The protoplasm of two decapitated sporangiophores is squeezed with tweezer tips into the middle of a small circle (3-5 mm in diameter) of Vaseline applied to a sterile cover glass. The ring of Vaseline prevents the extruded protoplasm from dispersing over the surface of the cover glass. Small amounts of Vaseline are applied on the corners of the cover glass, which is then inverted and placed over a deep depression slide containing one drop of sterile water on the bottom. This method facilitates the observation of the development of the regenerate from the mixed protoplasm under sterile conditions. The regenerated mycelia are transferred to agar medium in a petri dish.
Microinjection
Both donor and recipient sporangiophores, fixed with double-sided adhesive tape on a microscope slide or placed next to each other on an agar block, are cut off at their tips or basal ends. The cellular contents of the donor are aspirated into a micropipette fixed to a three-plane micromanipulator and injected into the recipient sporangiophore through the cut end. After 10-16 hours, provided sufficient humidity is maintained, new sporangiophores regenerate from the injected sporangiophore, and they are often heterokaryons with nuclei originating from both donor and recipient sporangiophores. Zalokar (1969) and Villet (1970, 1972) were successful with this method.
Sporangiophore Grafting
Burgeff (1912, 1914, 1924) discovered the phenomenon of heterokaryosis and made artificial heterokaryons by introducing the cut apex of one sporangiophore into the base of the other. A more effective method to obtain heterokaryons is to graft stage I sporangiophores at their apical ends (Ootaki 1973).
Two sturdy stage I sporangiophores containing a large amount of cytoplasm are removed from mycelia and laid facing each other on water-agar blocks placed on a microscope slide (Fig. 2) under a dissecting microscope.
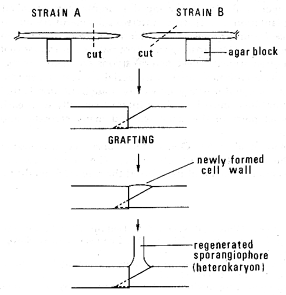
Figure 2. Procedure for grafting two stage I sporangiophores from genetically different strains in Phycomyces. The diagonally cut end of one sporangiophore is partially inserted into the open cut end of the other.
The facing tips of both sporangiophores are cut off with microscissors, one tip diagonally and the other transversely. The diagonally cut end of one sporangiophore is inserted slightly into the open end of the other by maneuvering the agar blocks. The glass slides carrying the grafts are then placed on a U-shaped glass-rod support and kept in a covered petri dish with a few milliliters of sterile distilled water to maintain humidity.
To achieve a high success rate, it is often helpful to remove the drop of exudate formed at the graft junction, 30-60 minutes after grafting, by touching with the closed tips of fine tweezers or with a small piece of filter paper. After 12-24 hours, regeneration occurs in the form of new sporangiophores at the graft union. These regenerates are often heterokaryotic; when the grafted sporangiophores adhere at the graft junction and regenerate a single sporangiophore, the success rate approaches 100% (Fig. 3).

Figure 3. A heterokaryotic sporangiophore regenerated singly at union of graft between two piloboloid mutants. These pairs failed to complement each other.
To induce the regeneration of a single sporangiophore at the graft junction, one may (1) cut and graft the sporangiophores at a relatively basal position (5-10 mm behind the apex) and (2) insert the diagonally cut end of one sporangiophore partially into the other. This method was used to make heterokaryons among color mutants (Ootaki et al. 1973; Torres-Martínez et al. 1980), phototropic mutants (Ootaki et al. 1974, 1977), deazariboflavin-resistant mutants (Delbruck and Ootaki 1979), and morphological pil mutants (Koga and Ootaki 1983) for genetic analyses.
Sexual-structure Transplantation
Gauger (1977) and Gauger et al. (1980) developed a simple method called sexual-structure transplantation to construct intersexual heterokaryons in Rhizopus and Phycomyces. Young zygotes are transferred with tweezers from the mating plate to fresh potato-dextrose agar, where patches of intersexual heterokaryotic mycelia often appear. These heterokaryons are readily recognized by their few sporangiophores, many tiny aerial contorted pseudophores, shaggy mycelial mat, and bright yellow color. One limitation is that the two strains to be combined must be able to interact sexually with each other, which excludes all pairs of the same sex and many carotene and developmental mutants.
Goodgal (see Bergman et al. 1969; Ootaki 1973) observed intersexual heterokaryons when he macerated the "soft tissue," (i.e., intimately enmeshed hyphae in the contact zone) and transferred them to fresh medium.
REFERENCES
Bergman, K., P.V. Burke, E. Cerdá-Olmedo, C.N. David, M. Delbrück, K.W. Foster, E.W. Goodell, M. Heisenberg, G. Meissner, M. Zalokar, D.S. Dennison, and W. Shropshire, Jr. 1969. Phycomyces. Bacteriol. Rev. 33: 99.
Binding, H. and H.J. Weber. 1974. The isolation, regeneration and fusion of Phycomyces protoplasts. Mol. Gen. Genet. 135: 273.
Blakeslee, A.F. 1906. Zygospore germinations in the Mucorineae. Syndowia Ann. Mycol. 4: 1.
Burgeff, H. 1912. Ueber Sexualität, Variabilität und Vererbung bei Phycomyces nitens. Ber. Detsch. Bot. Ges. 30: 679.
Burgeff, H. 1914. Untersuchungen über Variabilität, Sexualität und Erblichkeit bei Phycomyces nitens Kunze I. Flora 107: 259.
Burgeff, H. 1924. Untersuchungen über Sexualität und Parastismus bei Mucorineen, I. Bot. Abh. 4: 1.
Burgeff, H. 1928. Variabilität, Vrerbung und Mutation bei Phycomyces blakeskeeanus BGFF. Z. Vererbungsl. 49: 26.
Delbrück, M. and T. Ootaki. 1979. An unstable nuclear Gene in Phycomyces. Genetics 92: 27.
Gauger, W.L. 1977. Meiotic gene segregation in Rhizopus stolonifer. J. Gen. Microbiol. 101: 211.
Gauger, W., M.I. Peláez, M.L Alvarez, and A.P. Eslava. 1980. Mating type heterokarions in Phycomyces blakeskeeanus. Exp. Mycol. 4: 56
Heisenberg, M. and E. Cerdá-Olmedo. 1968. Segregation of heterokarions in the asexual cycle of Phycomyces. Mol. Gen. Genet. 102: 187.
Koga, K. and T. Ootaki. 1983. Complementation analysis among piloboloid mutants of Phycomyces blakesleeanus. Exp. Mycol. 7: 161.
Ootaki T. 1973. A new method for heterokaryon formation in Phycomyces. Mol. Gen. Genet. 121: 49.
Ootaki, T., E.P. Fischer, and P. Lockhart 1974. Complementation between mutants of Phycomyces with abnormal phototropism. Mol. Gen. Genet. 131: 233.
Ootaki, T., T. Kinno, K. Yoshida, and A.P. Eslava. 1977. Complementation between Phycomyces mutants of mating type (+) with abnormal phototropism. Mol. Gen. Genet. 152: 245.
Ootaki, T., A.C. Lighty, M. Delbrück, and W.-J Hsu. 1973. Complementation between mutants of Phycomyces deficient with respect to carotenogenesis. Mol. Gen. Genet. 121: 57.
Orban, G. 1919. Untersuchungen über die Sexualität von Phycomyces nitens. Beih. Bot. Centralbl. 36: 1.
Suárez, T., M. Orejas, and A.P. Eslava. 1985. Isolation, regeneration, and fusion of Phycomyces blakesleeanus spheroplasts. Exp. Mycol. 9: 203.
Torres-Martínez, S., F.J. Murillo, and E. Cerdá-Olmedo. 1980. Genetics of lycopene cyclization and substrate transfer in beta-carotene biosynthesis in Phycomyces. Genet. Res. 36: 299.
Villet, R.H. 1970. Genetic curing of blindness in Phycomyces blakesleeanus: A quantitative assessment of dominance. Nature 225: 453.
Villet, R.H. 1972. Microinjection of Phycomyces. Selection of a strain for possible biological assay of photoreceptor pigment. Plant Physiol. 49: 273.
Weide, A. 1939. Beobachtungen an Plasmaexplantaten von Phycomyces. Arch. Exp. Zellforsch. 23: 299.
Zalokar, M. 1969. Intracellular centrifugal separation of organelles in Phycomyces. J. Cell Biol. 41: 494.